如何加快产品开发流程,更快地交付创新产品?
通过支持集成式多域和多物理场工作流程的仿真和测试解决方案,打破学科之间的孤岛,尽早了解产品性能。
人工智能 (AI) 和电气化等尖端技术的影响力越来越大,加上不断变化的法规和消费者对可持续、个性化和智能产品的期望不断提高,加剧了现实世界工程挑战的复杂性。为了取得成功,公司需要有效地整合工程领域、方法和工具,同时解决熟练工程师的短缺问题。以仿真和测试为核心的综合数字孪生对于转变工程以快速开发、优化新概念并将其推向市场至关重要。
66% 的公司表示,使用仿真可以更早地获得见解。(Tech-Clarity)
73% 的顶级公司在产品开发中采用基于模型的系统工程,以解决跨多个工程领域的复杂设计需求。(Lifecycle insight)
79% 的领先公司促进了仿真分析师和测试工程师之间的协作。他们使用测试结果验证仿真模型,以提高准确性。(Tech-Clarity)
通过支持集成式多域和多物理场工作流程的仿真和测试解决方案,打破学科之间的孤岛,尽早了解产品性能。
工程仿真软件提供对产品性能的早期洞察,帮助优化设计并促进基于模型的系统工程。这样可以加快产品上市时间,提高投资回报率,并在工程和数字化转型方面具有竞争优势。
使用 Simcenter 工程仿真软件解锁数字孪生。通过建模和仿真、多学科设计探索、物理测试和仿真过程以及数据管理,在早期和整个设计周期中预测产品行为。

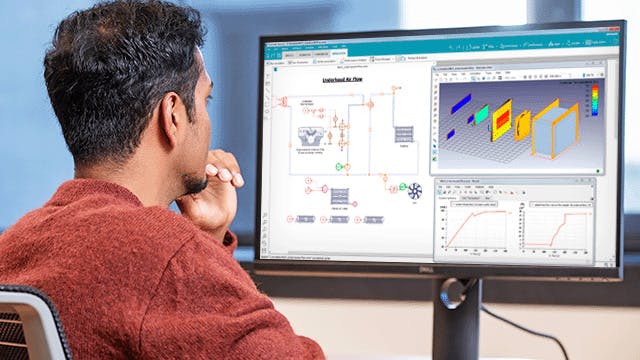

通过在项目早期采用仿真,我们显著降低了前端的成本和时间,因为我们在极短的时间内获得了正确的设计答案。这非常重要,因为我们可以让人们做出正确的设计决策,从而将开发时间缩短 50% 到 80%。
与 工程咨询服务 合作,帮助您构建工程数字孪生。
了解 Siemens Xcelerator Academy 如何帮助您掌握 Simcenter 并快速提高生产力。
加入我们的 Simcenter 专家 社区,扩展您的知识!
工程仿真软件使工程师能够在设计过程的早期深入了解产品行为,识别潜在问题并迭代设计,以提高性能、可靠性和效率。它在 、 、能源、 和制造等各个行业的产品开发、降低成本和推动创新方面发挥着至关重要的作用。
在设计周期的早期,仿真可以帮助消除不可行的设计。仿真还有助于探索更多创新设计方案,而对于此类设计方案,若非借助仿真手段,工程师往往不会予以考虑。对于使用仿真的设计工程师来说,有很多好处,包括节省成本和时间、更轻松的创新和提高产品质量。渐进式方法包括仿真分析师针对特定设计问题设置经过验证的仿真模板。然后,这些仿真模板可以作为引导式仿真工作流程的一部分进行集成,供设计师和设计工程师使用。适当的仿真数据管理也是仿真民主化不可或缺的一部分。
自动链接设计模型、仿真模型和分析结果的数据管理功能消除了哪些结果与哪些设计相关的歧义,从而提高了决策的信心。但是,对工件和文件进行跟踪和链接还不够。
决策者必须还能访问这些设计探索数据并从中快速提取可行性见解。结果可视化工具、报告和数据分析有助于工程师选择可满足所有需求的优化设计。
Simcenter 产品组合是Siemens Xcelerator的一部分,是一套灵活、开放和可扩展的前沿工程仿真和测试解决方案及相关服务。Simcenter 独特地将强大的多物理场工程方法与系统仿真、CAE仿真和物理测试相结合。它利用 AI 功能来加快决策速度并改善用户体验,并通过跨域工作流程自动化以及流程和数据管理提高生产力。
Simcenter 为工程师提供了对其产品或流程实际性能的详细见解,使他们能够在整个产品生命周期内提高生产力、缩短上市时间并加速创新。Simcenter 解决方案对于提供连接物理世界和虚拟世界的全面数字孪生是不可或缺的。
要了解和提供跨域性能,需要仿真和测试解决方案,这些解决方案可以部署在所有开发阶段以及所有性能领域和物理场中。此外,这些活动需要相互关联,而不是独立进行,以捕捉现实世界的多物理场现象并平衡相互冲突的属性。Simcenter 提供一流的预测仿真、测试和设计探索解决方案的集成组合,涵盖产品开发过程的所有阶段,以解决工程中棘手的问题,通过提高生产力和赋能创新来帮助实现工程转型。
仿真和测试不能再被视为孤立的活动。数字孪生在整个生命周期中提供价值,需要对其演进进行管理,以实现可追溯性和变化的影响。这只有通过提供仿真和测试作为整体数字产品开发计划的一部分才能实现。Siemens Xcelerator 模糊了电气、机械和软件等工程领域之间的界限, Simcenter 仿真和测试解决方案与设计、实现和优化生命周期的各个阶段 Siemens Xcelerator的解决方案相辅相成。
在数字化转型的征程中,企业需要可靠的合作伙伴,并拥有丰富的资源,以不断推动工具和应用程序的创新。西门子在研发方面投入了大量资金,利用与邻近地区知名公司的合作伙伴关系,并投资于拥有可以改变未来业务的技术的初创公司。所有这些都确保了源源不断的创新,可以帮助我们的客户保持领先地位,并比竞争对手更快地实现数字化的好处。Simcenter 工程和咨询服务提供深厚的领域知识、设施、资源和专业知识,以加速工程流程转型并获得投资回报。