此页面使用自动翻译以中文显示。
改为用英语查看?
此翻译是否有帮助?
改变日常生活
共同改造行业,将创意转化为创新。
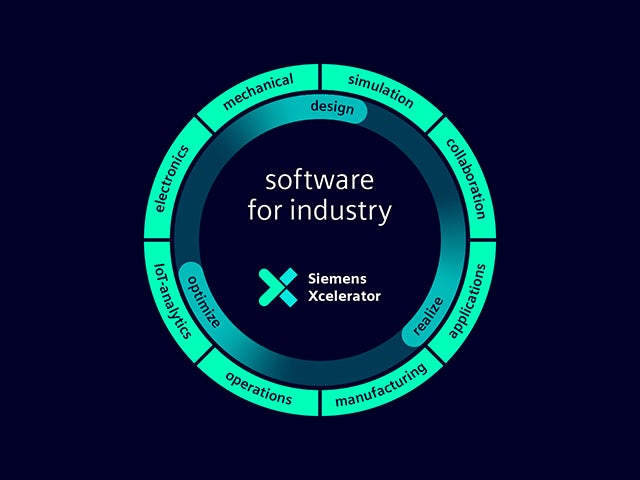
怎样才能成为数字化企业?
Surf Loch 正在使用当今的数字化技术来改变冲浪行业。他们拥有一支由专业工程师组成的小团队,使用 Siemens Xcelerator 产品组合中的软件和服务,让所有冲浪者都有机会随时乘风破浪。
详细了解他们鼓舞人心的数字化转型之旅。
SIEMENS XCELERATOR
改变日常生活
请访问我们的博客,了解有关 Siemens Xcelerator 产品组合和 Siemens Xcelerator 即服务的更多信息。