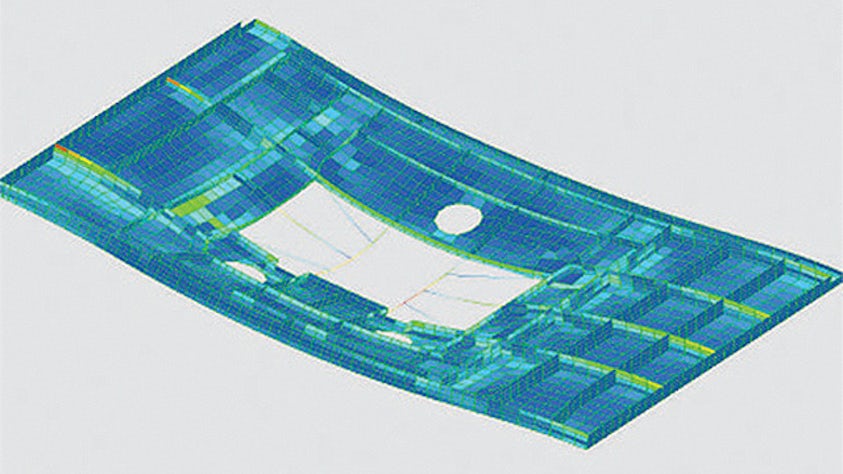
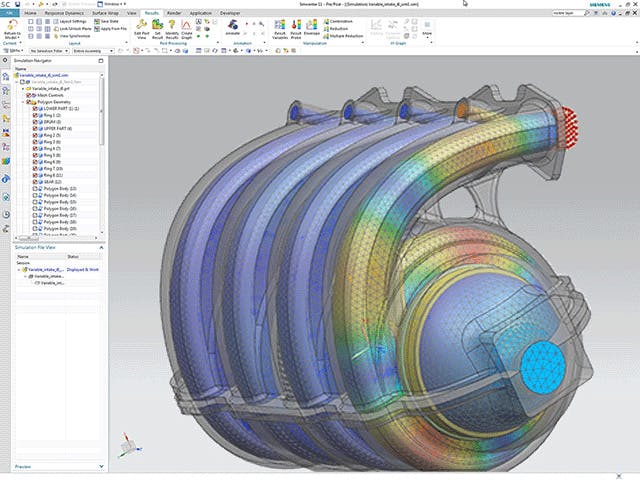
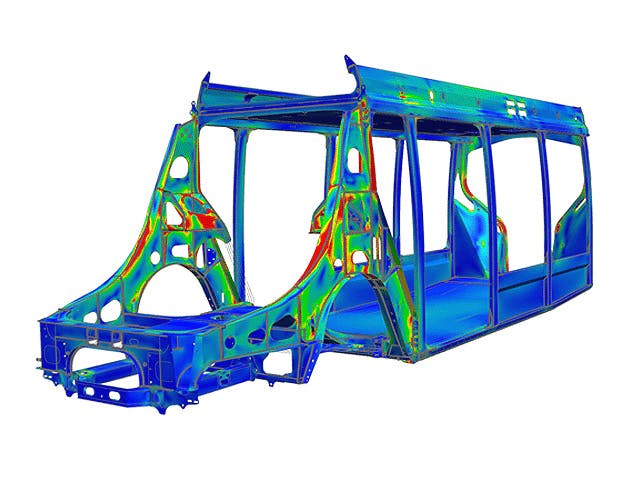
To stay ahead in the innovation race, engineers need to be able to quickly predict the outcome of design changes on the real-world performance of their product. Engineering simulation provides an excellent way for you to cost-effectively evaluate how your products will perform under expected operating conditions.
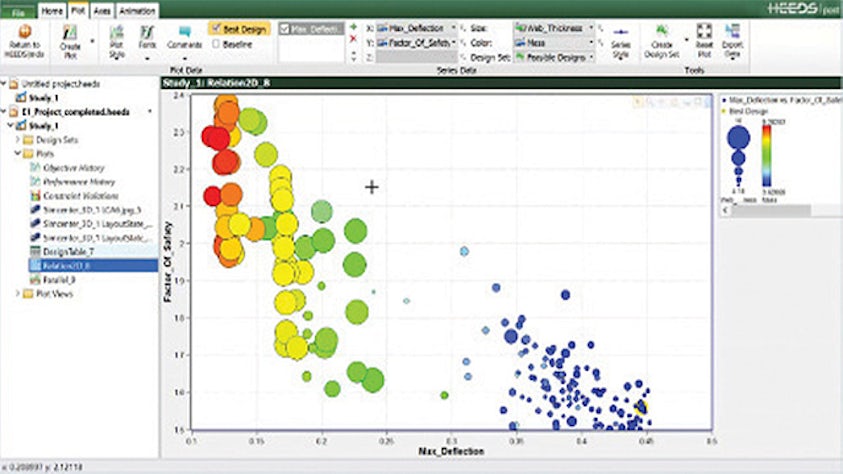
Design exploration and optimization solutions take simulation to the next level yielding new, innovative product designs that result in exceptional performance.
Improving cooling of gas turbines with design space exploration
Learn how Kawasaki Heavy Industries and B&B-Agema GmbH improved the cooling effectiveness of their turbines.