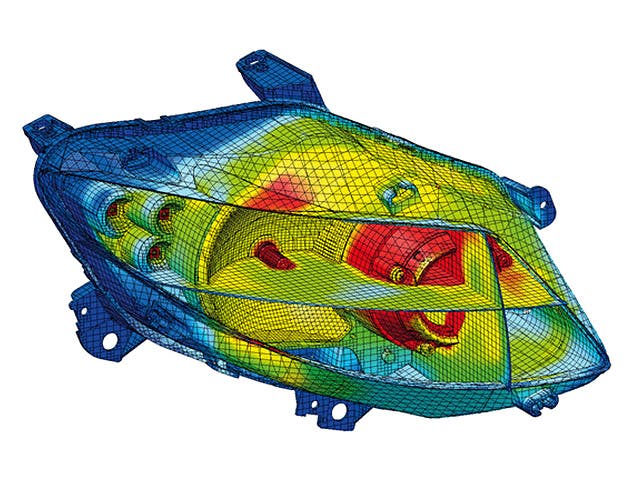
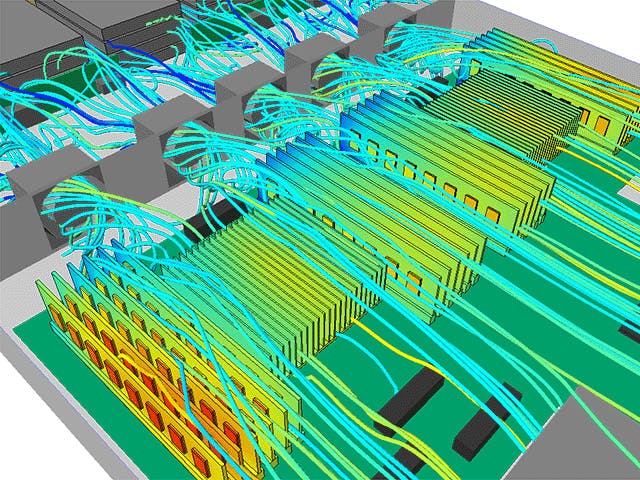
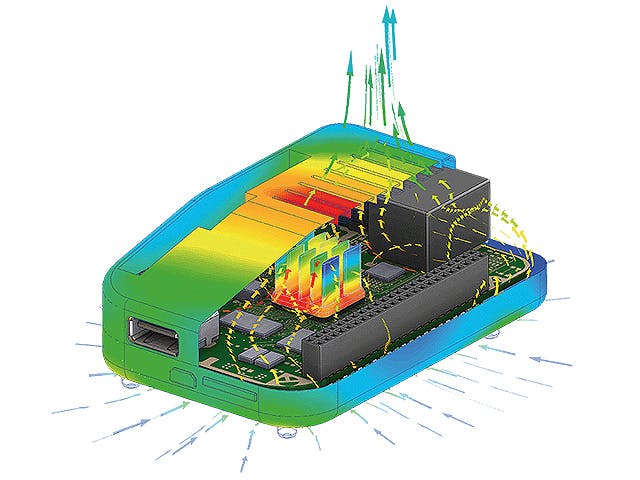
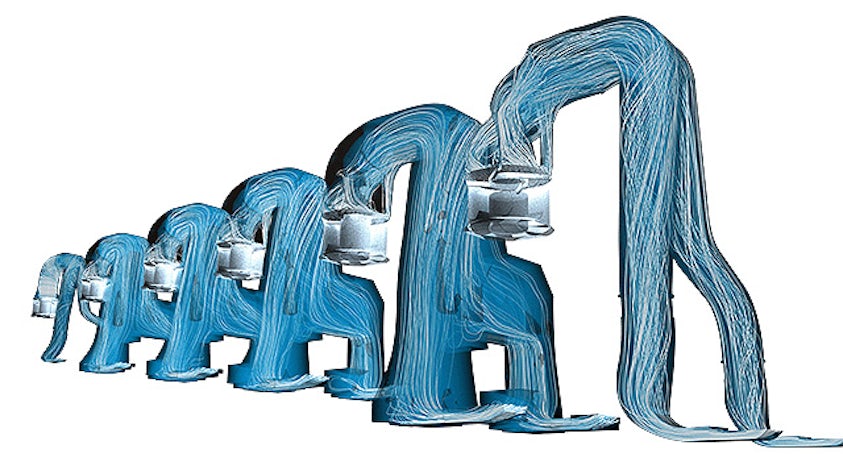
The real-world performance of many products depends on their interaction with fluids, either gases, liquids or a combination of both. Computational fluid dynamics simulation offers the ability to simulate and predict flow and heat transfer behavior. However, in a world of ever-increasing product complexity, understanding a product’s fluid dynamics and heat transfer performance in an isolated manner is no longer sufficient.
To turn the complexity into a competitive advantage, today’s CFD engineers need to be able to model a wide range of fluid-related physics, from reacting flows to aeroacoustics, from multiphase flows to particle dynamics, from electronics cooling to aerodynamics.
Simcenter fluids and thermal simulation software allow you to predict the most complex fluid dynamics problems virtually and turn your insights into product innovation.