Transformacja codzienności
Wspólnie przekształcamy przemysł oraz przekuwamy pomysły w innowacyjne rozwiązania.
Jak sztuczna inteligencja zmienia konserwację fabryk
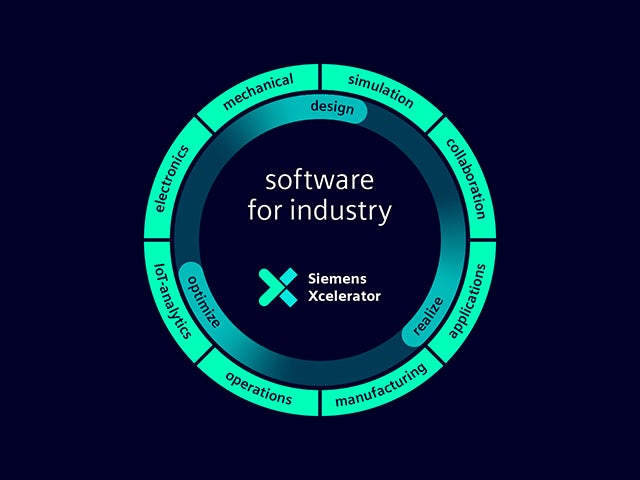
Nasz cyfrowy bliźniak jest jedynym źródłem zaufania, które umożliwia podejmowanie mądrych decyzji w oparciu o dane. Tylko rozwiązania firmy Siemens łączą świat rzeczywisty i cyfrowy, umożliwiając powstanie prawdziwe cyfrowego przedsiębiorstwa.
Zrównoważony rozwój
Tworzymy zrównoważone innowacje przemysłowe z myślą o świecie, w którym chcemy żyć dziś i jutro, oraz przemyślnie niepozostawiającym trwałych śladów na naszej planecie.
Przyszłość mobilności
Umożliwiamy przyszłość mobilności w powietrzu, na lądzie i na morzu, przyczyniając się do rewolucji w transporcie mającej na celu zwiększenie komfortu podróżowania i zmniejszenie jego negatywnych skutków dla środowiska.
Wytwarzanie addytywne
Oferujemy przedsiębiorstwom każdej wielkości wdrożenie pragmatycznego wytwarzania addytywnego. Umożliwiamy uruchomienie kompleksowych procesów wytwarzania addytywnego, opracowanych z myślą o łatwej obsłudze, efektywności kosztowej i powszechnym stosowaniu.
Sztuczna inteligencja
Wykorzystujące duże zbiory danych nasze innowacje i rozwiązania w zakresie sztucznej inteligencji umożliwiają podejmowanie bardziej przemyślanych decyzji i osiągnięcie większej wydajności, lepszej jakości oraz krótszego czasu wprowadzania produktów na rynek, a także otwierają przed przedsiębiorstwami nowe możliwości działania.
Projektowanie systemów w oparciu o modele
Nasza metodologia projektowania systemów w oparciu o zintegrowane modele (MBSE) ujednolica wszystkie dziedziny potrzebne do tworzenia dzisiejszych inteligentnych systemów i wszystkie koncentrują się na przyspieszeniu innowacji produktowych lub sukcesu biznesowego.
Co należy zrobić, aby stać się przedsiębiorstwem cyfrowym?
Firma Surf Loch wykorzystuje technologię cyfrową, aby zrewolucjonizować branżę surfingową. Niewielki zespół zaangażowanych inżynierów wykorzystał oprogramowanie i usługi dostępne w portfolio Xcelerator firmy Siemens, aby umożliwić wszystkim surferom możliwość wykonania idealnego ślizgu na fali.
Dowiedz się więcej o ich inspirującej transformacji cyfrowej.
Transformacja codzienności
Odwiedź nasz blog, aby znaleźć więcej informacji na temat portfolio Siemens Xcelerator i Siemens Xcelerator as a Service.