이 페이지는 자동 번역을 사용하여 한국어로 표시됩니다.
영어로 보시겠습니까?
이 번역이 도움이 되었나요?
일상을 혁신합니다.
더불어 산업을 혁신하면서 혁신적인 아이디어를 실현합니다.
AI가 공장 유지 보수를 변화시키는 방법
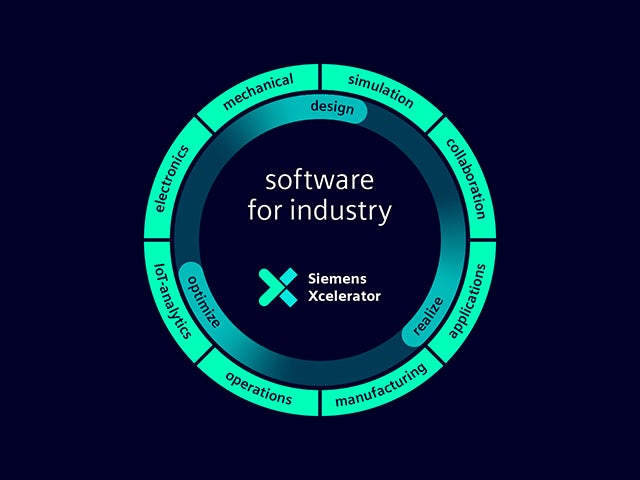
디지털 트윈은 데이터를 기반으로 현명한 결정을 내릴 수 있는 신뢰할 수 있는 단일 소스입니다. Siemens만이 실제 환경과 디지털 환경을 결합하여 진정한 디지털 엔터프라이즈를 구축합니다.
적층 제조
당사는 규모에 관계없이 모든 회사에 실용적인 적층 제조를 제공합니다. 당사는 적층 제조를 위한 엔드투엔드 프로세스를 지원하여 간단하고 비용 효율적이며 널리 확산될 수 있게 합니다.
인공 지능
당사의 AI 혁신 및 솔루션은 빅데이터를 활용하여 보다 스마트한 의사 결정, 효율성 향상, 품질 향상, 시장 출시 시간 단축을 지원하는 동시에 새로운 비즈니스를 창출합니다.
모델 기반 시스템 설계
슈나이더 일렉트릭의 통합 모델 기반 시스템 설계(MBSE) 방법론은 오늘날의 스마트 시스템을 만드는 데 필요한 모든 영역을 통합하며, 모두 제품 혁신 또는 비즈니스 성공을 가속화하는 데 중점을 두고 있습니다.
디지털 엔터프라이즈 구현을 위해 필요한 것은 무엇입니까?
Surf Loch는 디지털 기술을 사용하여 서핑 산업을 혼란에 빠뜨리고 있습니다. 전담 엔지니어로 구성된 소규모 팀과 함께 Siemens Xcelerator 포트폴리오 내의 소프트웨어와 서비스를 사용하여 모든 서퍼가 원할 때마다 완벽한 파도를 탈 수 있는 기회를 제공합니다.
영감을 주는 디지털 트랜스포메이션 여정에 대해 자세히 알아보세요.
SIEMENS XCELERATOR
일상을 혁신합니다.
Siemens Xcelerator 포트폴리오 및 Siemens Xcelerator as a Service에 대한 자세한 내용은 블로그를 참조하십시오.