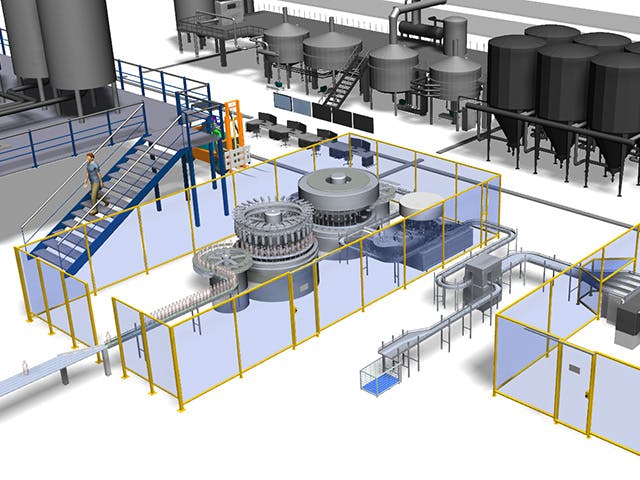
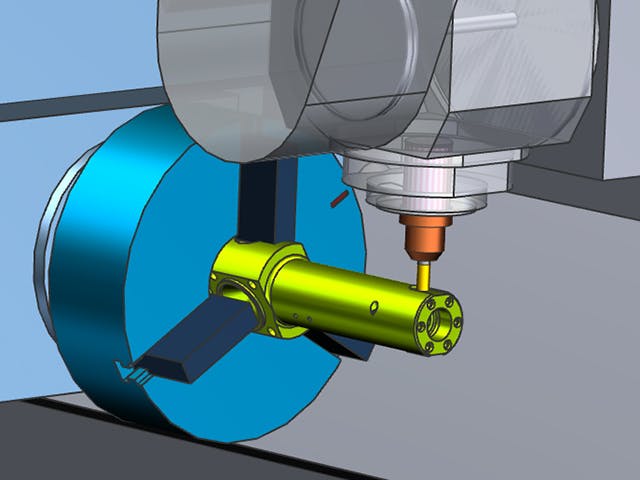
デジタル・マニュファクチャリングとは、シミュレーション、3Dビジュアライゼーション、分析、コラボレーション・ツールで構成される統合されたコンピューターベースのシステムを使用して、製品と製造プロセスの定義を同時に作成することです。デジタルマニュファクチャリングは、製造可能性を考慮した設計(DFM)、コンピューター統合製造(CIM)、フレキシブルマニュファクチャリング、リーン生産などの製造イニシアチブから進化し、製品とプロセスの共同設計の必要性を強調しました。
製品ライフサイクル管理(PLM)がもたらす長期的なメリットの多くは、包括的な デジタル・マニュファクチャリング戦略なしには実現できません。デジタル・マニュファクチャリングは、PLMと製造現場のアプリケーションや機器を統合する重要なポイントであり、設計グループと製造グループの間で製品関連の情報を交換できます。この連携により、製造企業は市場投入までの時間と数量の目標を達成し、コストのかかる下流工程の変更を減らすことでコスト削減を実現できます。
関連製品: Tecnomatix |NX |Solid Edge