Rivoluzionare il quotidiano
Rivoluzionare il settore e trasformare le idee in progetti innovativi.
In che modo l'intelligenza artificiale sta cambiando la manutenzione delle fabbriche
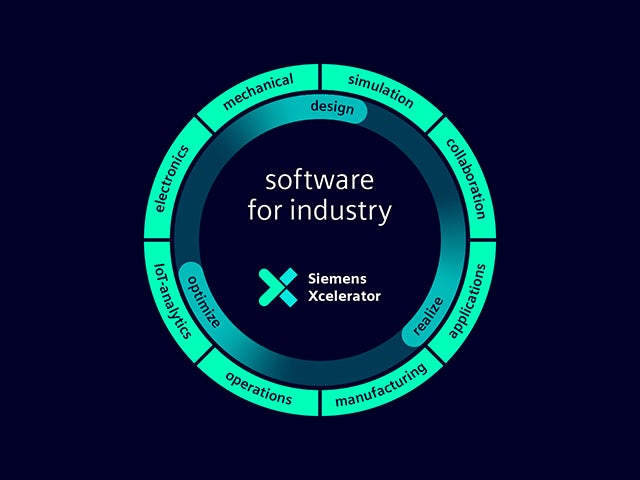
Il nostro gemello digitale è l'unica fonte di fiducia per consentire decisioni intelligenti basate sui dati. Soltanto Siemens combina il mondo reale e quello digitale per creare una vera Digital Enterprise.
Sostenibilità
Creiamo innovazione industriale sostenibile per il mondo in cui vogliamo vivere, oggi e in futuro, e per un'industria che si impegna a non lasciare tracce su questo pianeta.
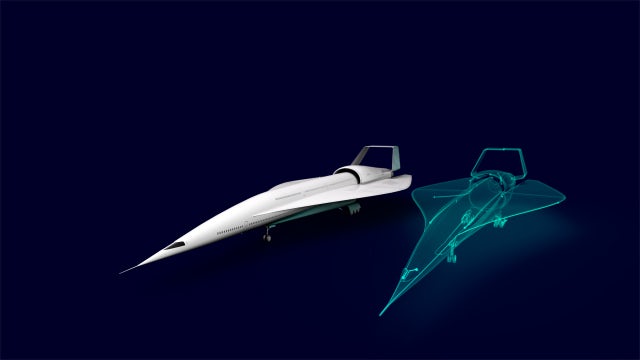
Il futuro della mobilità
Stiamo lavorando al futuro della mobilità aerea, terrestre e marittima, contribuendo a rivoluzionare il settore dei trasporti per rendere i viaggi più piacevoli e sostenibili.
Additive manufacturing
Forniamo soluzioni di additive manufacturing per aziende di tutte le dimensioni. Abilitiamo i processi end-to-end per la produzione additiva, al fine di renderla più semplice ed economica.
Intelligenza artificiale
Sfruttando i Big Data, le nostre innovazioni e soluzioni di intelligenza artificiale consentono un processo decisionale più intelligente, una maggiore efficienza, una migliore qualità e un time-to-market più rapido, sbloccando al contempo nuove attività.
Progettazione di sistemi basati su modelli
La nostra metodologia MBSE (Integrated Model-Based System Design) unifica tutti i domini necessari per creare i sistemi intelligenti di oggi e sono tutti focalizzati sull'accelerazione dell'innovazione dei prodotti o del successo aziendale.
Come diventare un'impresa digitale?
Surf Loch utilizza la tecnologia digitale per rivoluzionare l'industria del surf. Con un piccolo team di ingegneri dedicati, l’azienda ha utilizzato i software e servizi all'interno del portfolio Siemens Xcelerator per permettere a tutti i surfisti di cavalcare l'onda perfetta ogni volta che lo desiderano.
Scopri di più sul loro percorso di trasformazione digitale
Rivoluzionare il quotidiano
Visita il nostro blog per trovare maggiori informazioni sul portafoglio Siemens Xcelerator e Siemens Xcelerator as a Service.