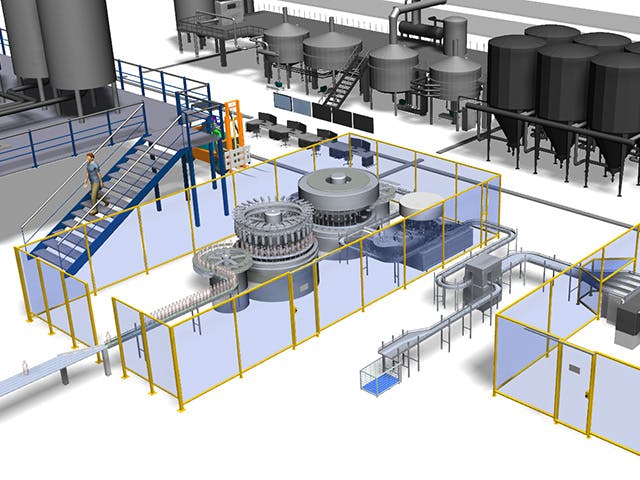
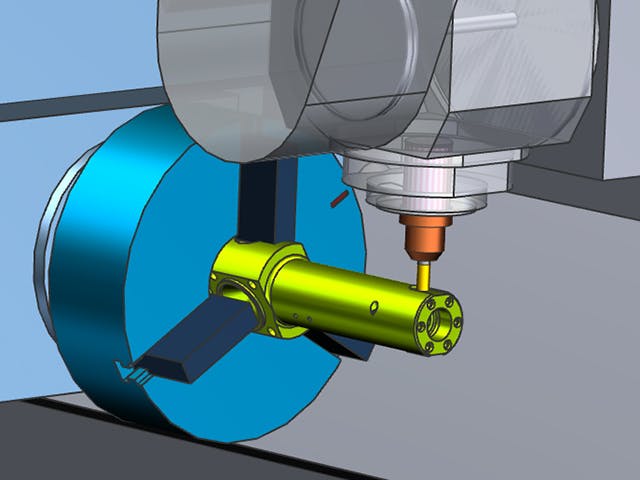
La fabrication numérique est l’utilisation d’un système informatique intégré composé d’outils de simulation, de visualisation 3D, d’analyse et de collaboration pour créer simultanément des définitions de produits et de processus de fabrication. La fabrication numérique a évolué à partir d’initiatives de fabrication telles que la conception pour la fabricabilité (DFM), la fabrication intégrée par ordinateur (CIM), la fabrication flexible et la fabrication allégée qui mettent en évidence la nécessité d’une conception collaborative des produits et des processus.
De nombreux avantages à long terme de la gestion du cycle de vie des produits (PLM) ne peuvent être obtenus sans une stratégie de fabrication numérique complète. La fabrication numérique est un point clé d’intégration entre les applications et les équipements PLM et d’atelier, permettant l’échange d’informations relatives aux produits entre les groupes de conception et de fabrication. Cet alignement permet aux entreprises manufacturières d’atteindre leurs objectifs de délai de mise sur le marché et de volume, ainsi que de réaliser des économies de coûts en réduisant les changements coûteux en aval.
Produits connexes : Tecnomatix | NX | Solid Edge