Transformer le quotidien
Transformer l'industrie ensemble et concrétiser les idées.
Comment l’IA change la maintenance des usines
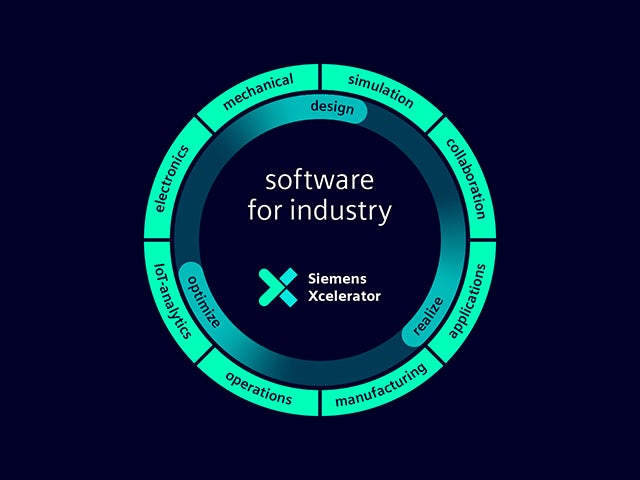
Notre jumeau numérique est la source unique de confiance pour prendre des décisions intelligentes basées sur les données. Siemens est la seule entreprise à allier le monde réel et le monde virtuel pour bâtir une véritable entreprise numérique.
Durabilité
Nous proposons des solutions industrielles innovantes et durables adaptées aux besoins du monde d'aujourd'hui et de demain, ainsi qu'à ceux des entreprises qui souhaitent préserver la planète.
L’avenir de la mobilité
Nous nous engageons pour l'avenir de la mobilité aérienne, terrestre et maritime en fournissant les outils qui aideront les entreprises à révolutionner le transport.
Fabrication additive
Nous proposons une fabrication additive pragmatique pour les entreprises de toutes tailles. Nous mettons en œuvre des processus de bout en bout pour faciliter la fabrication additive, la rendre rentable et la généraliser.
Intelligence artificielle
En tirant parti du Big Data, nos innovations et solutions d’IA permettent de prendre des décisions plus intelligentes, de gagner en efficacité et d'améliorer la qualité des produits afin de les lancer plus rapidement.
Conception de systèmes basée sur des modèles
Notre méthodologie intégrée de conception de systèmes basée sur des modèles (MBSE) unifie tous les domaines nécessaires à la création des systèmes intelligents d’aujourd’hui et est axée sur l’accélération de l’innovation des produits ou de la réussite commerciale.
Que faut-il pour devenir une entreprise numérique ?
Surf Loch surf sur la vague du numérique pour transformer son secteur d'activité. Une petite équipe d'ingénieurs passionnés a utilisé les logiciels et les services du portefeuille Siemens Xcelerator pour que tous les surfeurs aient la possibilité de prendre la vague parfaite quand ils le souhaitent.
Découvrez leur parcours de transformation numérique.
Transformer le quotidien
Visitez notre blog pour plus d’informations sur la gamme Siemens Xcelerator et Siemens Xcelerator as a Service.