Siemens ofrece soluciones de ingeniería de software embebido tanto para automoción como para software embebido . Siemens ha dejado de ofrecer software integrado independiente para SoC con la retirada en noviembre de 2023 de los productos Nucleus, Nucleus Hypervisor, Nucleus ReadyStart, Sokol Flex Linux, Sokol Omni Linux y Sourcery CodeBench (incluidos los complementos asociados). Los contratos de soporte existentes para estos productos aún se están cumpliendo, comuníquese con el Centro de soporte de Siemens para obtener más información.
¿Cuáles son los diferentes tipos de software embebido y sus propósitos?
- Sistema operativo: un sistema operativo (SO), en su sentido más general, es un software que permite a un usuario ejecutar otras aplicaciones en un dispositivo informático. El sistema operativo administra los recursos de hardware de un procesador, incluidos los dispositivos de entrada, como el teclado y el ratón, los dispositivos de salida, como pantallas o impresoras, las conexiones de red y los dispositivos de almacenamiento, como los discos duros y la memoria. El sistema operativo también proporciona servicios para facilitar la ejecución y gestión eficientes de los programas de aplicación de software, así como la asignación de memoria para ellos.
- Firmware: el firmware es un tipo de software que se escribe directamente para una pieza de hardware. Funciona sin pasar por las API, el sistema operativo o los controladores de dispositivos, lo que proporciona las instrucciones y la orientación necesarias para comunicarse con otros dispositivos o realizar tareas y funciones básicas según lo previsto.
- Middleware: el middleware es una capa de software situada entre las aplicaciones y los sistemas operativos. El middleware se utiliza a menudo en sistemas distribuidos donde simplifica el desarrollo de software al proporcionar lo siguiente:
- Ocultar las complejidades de las aplicaciones distribuidas
- Enmascarar la heterogeneidad del hardware, los sistemas operativos y los protocolos
- Proporcionar interfaces uniformes y de alto nivel que se utilizan para crear aplicaciones interoperables, reutilizables y portátiles.
- Ofrecer un conjunto de servicios comunes que minimice la duplicación de esfuerzos y mejore la colaboración entre aplicaciones
- Aplicación: el usuario final desarrolla la aplicación de software final que se ejecuta en el sistema operativo, utiliza o interactúa con el middleware y el firmware, y es el foco principal de la función de destino de los sistemas integrados. Cada aplicación final es única, mientras que los sistemas operativos y el firmware pueden ser idénticos de un dispositivo a otro.
Software embebido vs sistemas embebidos
Los componentes de hardware dentro de un dispositivo que ejecuta software integrado se denominan "sistema integrado". Algunos ejemplos de componentes de hardware utilizados en sistemas integrados son los circuitos de alimentación, las unidades centrales de procesamiento, los dispositivos de memoria flash, los temporizadores y los puertos de comunicación en serie. Durante las primeras fases de diseño de un dispositivo, se decide el hardware que conformará el sistema embebido y su configuración dentro del dispositivo. Luego, el software embebido se desarrolla desde cero para ejecutarse exclusivamente en ese hardware en esa configuración precisa. Esto hace que el diseño de software embebido sea un campo especializado que requiere un profundo conocimiento de las capacidades de hardware y programación de computadoras.
Ejemplos de funciones integradas basadas en software
Casi todos los dispositivos con placas de circuito y chips de computadora tienen estos componentes organizados en un sistema de software integrado. Como resultado, los sistemas de software integrados son omnipresentes en la vida cotidiana y se encuentran en toda la tecnología de consumo, industrial, automotriz, aeroespacial, médica, comercial, de telecomunicaciones y militar.
Algunos ejemplos comunes de funciones integradas basadas en software son:
- Sistemas de procesamiento de imágenes que se encuentran en los equipos de imágenes médicas
- Sistemas de control fly-by-wire que se encuentran en los aviones
- Sistemas de detección de movimiento en cámaras de seguridad
- Sistemas de control de tráfico que se encuentran en los semáforos
- Sistemas de temporización y automatización que se encuentran en los dispositivos domésticos inteligentes
¿Cuáles son los diferentes tipos de sistemas embebidos?
Cuando se basan en el rendimiento y los requisitos funcionales, hay cinco clases principales de sistemas integrados:
- Los sistemas embebidos en tiempo real completan las tareas de forma determinista y repetible, lo que se ve afectado por la arquitectura y la programación subyacentes de los sistemas operativos, así como por el rendimiento de los subprocesos, la bifurcación y la latencia de interrupción. Los sistemas embebidos de uso general no contienen un requisito en tiempo real y pueden administrar interrupciones o bifurcaciones sin depender de un tiempo de finalización. Las pantallas gráficas y la gestión del teclado y el ratón son buenos ejemplos de sistemas generales.
- Los sistemas integrados independientes pueden completar tareas sin un sistema host o recursos de procesamiento externos. Pueden emitir o recibir datos de dispositivos conectados, pero no dependen de ellos para completar su tarea.
- Los sistemas integrados independientes pueden completar su tarea sin un sistema host o recursos de procesamiento externos. Pueden emitir o recibir datos de dispositivos conectados, pero no dependen de ellos para completar su tarea.
- Los sistemas integrados en red dependen de una red conectada para realizar las tareas asignadas.
- En función de la complejidad de la arquitectura de hardware del sistema, existen tres tipos principales de sistemas embebidos: Los sistemas integrados en red dependen de una red conectada para realizar las tareas asignadas.
Cómo afectan los mercados finales a los sistemas embebidos
Los requisitos y componentes del sistema integrado diferirán según las demandas del mercado objetivo. Algunos ejemplos incluyen:
- Consumidor: en aplicaciones como bienes de consumo como lavadoras, dispositivos portátiles y teléfonos móviles, los sistemas integrados enfatizan el tamaño reducido del
- Sistema en chip, bajo consumo de energía o funcionamiento con batería, e interfaces gráficas. En estas aplicaciones se valoran los sistemas operativos configurables y la capacidad de apagar "dominios" no operativos del diseño.
- Redes: aplicaciones que permiten la conectividad, la comunicación, las operaciones y la administración de una red empresarial. Proporciona la ruta de comunicación y los servicios entre usuarios, procesos, aplicaciones, servicios y redes externas/Internet. Las aplicaciones de red integradas se centran en la velocidad de respuesta, el procesamiento de paquetes y las rutas de hardware periférico.
- Industrial: para aplicaciones como la gestión de fábricas, motores y molinos de viento, el énfasis tiende a asegurar la conectividad en la nube y el funcionamiento determinista en "tiempo real" y puede centrarse en gran medida en el middleware.
- Médica, automotriz y aeroespacial: estas industrias necesitan sistemas críticos de seguridad mixta, donde partes del diseño estén aisladas entre sí para garantizar que solo los datos necesarios entren o salgan del sistema (seguridad) y que no se cause daño al usuario final (seguridad). Algunos ejemplos son los sistemas de conducción autónoma en automóviles y dispositivos médicos. Estos sistemas embebidos pueden presentar una combinación de código abierto (Linux) y sistemas operativos deterministas en tiempo real (RTOS) y utilizan en gran medida middleware probado.
¿Por qué es diferente el software integrado para automóviles?
En la electrónica automotriz, se producen interacciones complejas en tiempo real a través de múltiples sistemas integrados que funcionan cada control, como frenado, dirección, suspensión, tren motriz, etc. La carcasa física que contiene cada sistema integrado se denomina unidad de control electrónico (ECU). Cada ECU y su software integrado forman parte de una arquitectura eléctrica compleja conocida como sistema distribuido.
Al comunicarse entre sí, las ECU que componen el sistema distribuido de un vehículo pueden ejecutar una variedad de funciones, como frenado automático de emergencia, control de crucero adaptativo, control de estabilidad, faros adaptativos y mucho más. Una sola función puede necesitar interacciones entre 20 o más aplicaciones de software integradas distribuidas en numerosas ECU conectadas por varios protocolos de red. Los complejos algoritmos de control implementados con el software integrado garantizan la sincronización adecuada de las funciones, las entradas y salidas necesarias y la seguridad de los datos.
Algunos ejemplos comunes de funciones basadas en aplicaciones de software para automóviles son:
- Funciones ADAS (sistemas avanzados de asistencia al conductor) como control de crucero adaptativo, frenado automático de emergencia, asistente de mantenimiento de carril, asistencia de tráfico, advertencias de cambio de carril
- Gestión de la batería
- Compensación de par
- Control de la tasa de inyección de combustible
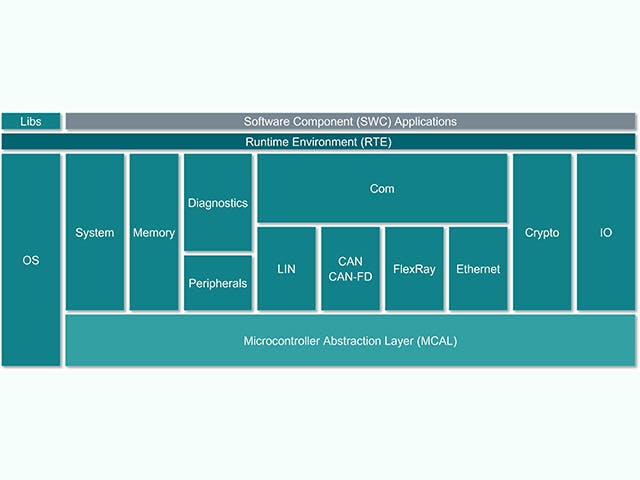
Pila de software de ECU
La unidad de control electrónico o ECU se compone de una unidad informática principal con hardware a nivel de chip y una pila de software integrado. Sin embargo, existe una tendencia creciente entre los fabricantes de automóviles a diseñar ECU con circuitos integrados complejos que contienen múltiples núcleos informáticos en un solo chip, lo que se conoce como sistema en un chip (SoC). Estos SoC pueden alojar una multitud de abstracciones de ECU para consolidar el hardware. La pila de software para una ECU suele incluir una gama de soluciones, desde firmware de bajo nivel hasta aplicaciones de software integradas de alto nivel.
Pila de ECU | Descripción |
Aplicación de software embebido | Algoritmos de control, procesamiento, servicios |
Marco de aplicación | Marcos de seguridad y protección |
Entorno operativo | AUTOSAR classic, AUTOSAR Adaptive, Entradas/Canales de salida |
Virtualizaciones integradas | Abstracciones de ECU y sistema operativo en tiempo real |
Firmware | Cargadores de arranque, almacenamiento seguro, subprocesos seguros |
Hardware | Dispositivos basados en silicio, microcontroladores, placas de una o varias capas |