Transforma lo cotidiano
Apostamos por transformar juntos la industria y convertir las ideas en innovación.
Cómo la IA está cambiando el mantenimiento de las fábricas
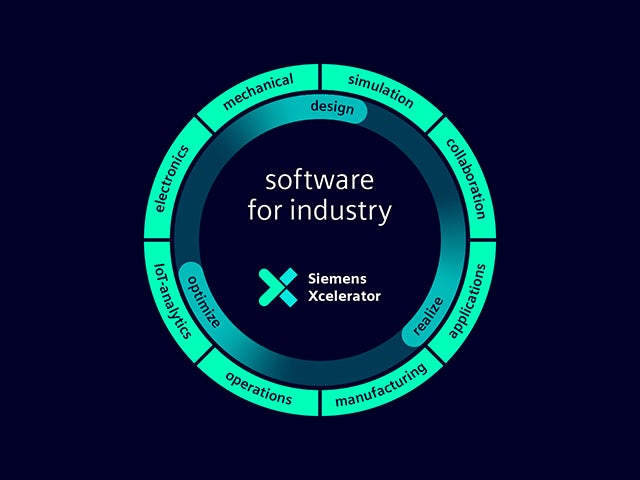
Nuestro gemelo digital es la única fuente de confianza para permitir decisiones inteligentes basadas en datos. Solo Siemens combina el mundo real con el digital para construir una verdadera empresa digital.
Sostenibilidad
Creamos innovación industrial sostenible para un mundo en el que queremos vivir, hoy y mañana, y para una industria que no desea dejar huella en este planeta.
El futuro de la movilidad
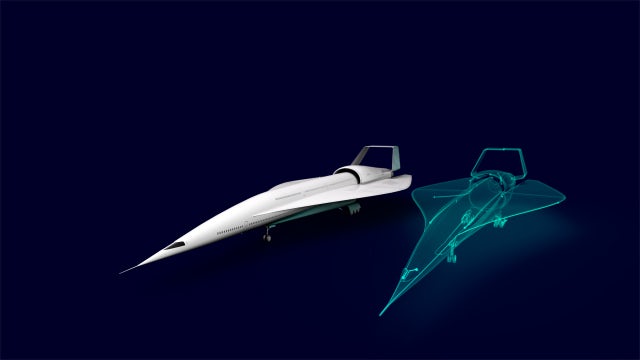
Estamos abriendo las puertas al futuro de la movilidad por aire, tierra y mar. Contribuimos a la revolución del transporte para hacer que los viajes sean más agradables y sostenibles.
Fabricación aditiva
Ofrecemos fabricación aditiva pragmática para empresas de todos los tamaños. Habilitamos procesos integrales para que la fabricación aditiva sea fácil, rentable y generalizada.
Inteligencia artificial
Al aprovechar el Big Data, nuestras innovaciones y soluciones de IA permiten una toma de decisiones más inteligente, una mayor eficiencia y calidad, y un tiempo de comercialización más rápido. Además, se abre la posibilidad a nuevos negocios.
Diseño de sistemas basados en modelos
Nuestra metodología integrada de diseño de sistemas basados en modelos (MBSE) unifica todos los dominios necesarios para crear los sistemas inteligentes actuales y todos se centran en acelerar la innovación de productos o el éxito empresarial.
¿Qué se necesita para convertirse en una empresa digital?
Surf Loch utiliza la tecnología digital actual para transformar la industria del surf. Con un pequeño equipo de ingenieros, aprovechó el software y los servicios del portfolio de Siemens Xcelerator para dar a todos los surfistas la oportunidad de surcar la ola perfecta cuando lo deseen.
Obtén más información sobre su inspirador proceso de transformación digital.
Transforma lo cotidiano
Visite nuestro blog para obtener más información sobre la cartera de Siemens Xcelerator y Siemens Xcelerator as a Service.