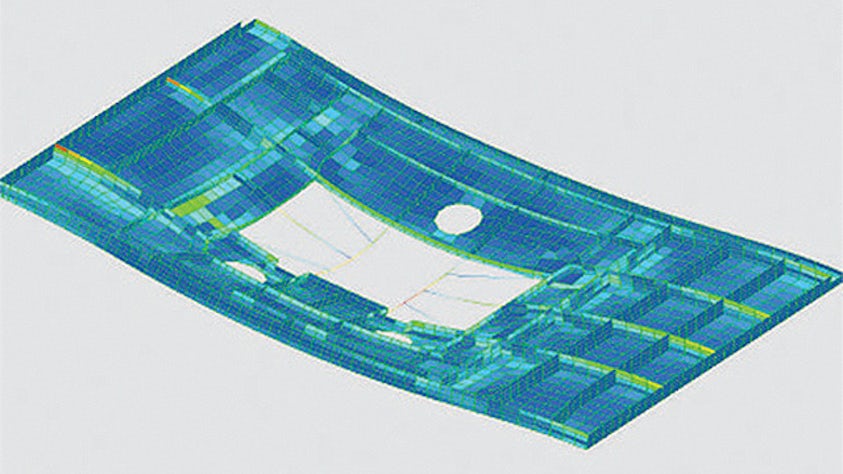
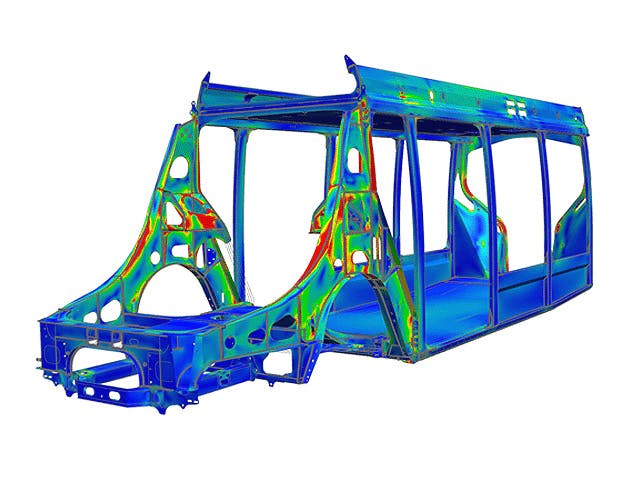
Um im Innovationswettbewerb einen Schritt voraus zu sein, müssen Ingenieure in der Lage sein, das Ergebnis von Konstruktionsänderungen auf die reale Performance ihres Produkts schnell vorherzusagen. Die Konstruktionssimulation ist eine hervorragende Lösung, um die Leistung Ihrer Produkte unter den erwarteten Betriebsbedingungen kostengünstig zu evaluieren.
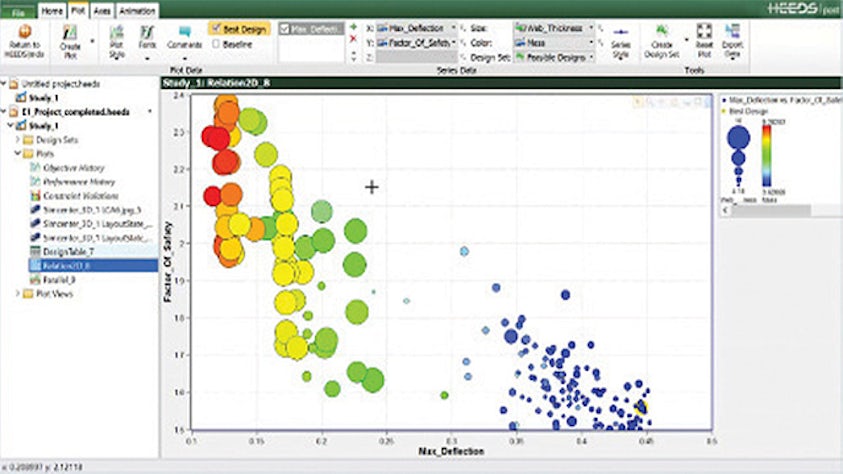
Design-Explorations- und Optimierungslösungen heben die Simulation auf eine neue Qualitätsstufe und führen zu neuen, innovativen Produktentwicklungen, die zu einer außergewöhnlichen Leistung führen.
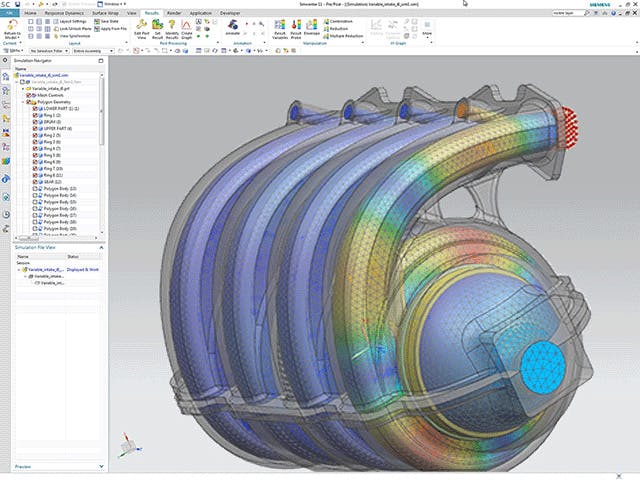
Verbesserung der Kühlung von Gasturbinen durch Erkundung des Konstruktionsraums
Erfahren Sie, wie Kawasaki Heavy Industries und die B&B-Agema GmbH die Kühlwirkung ihrer Turbinen verbessern konnten.