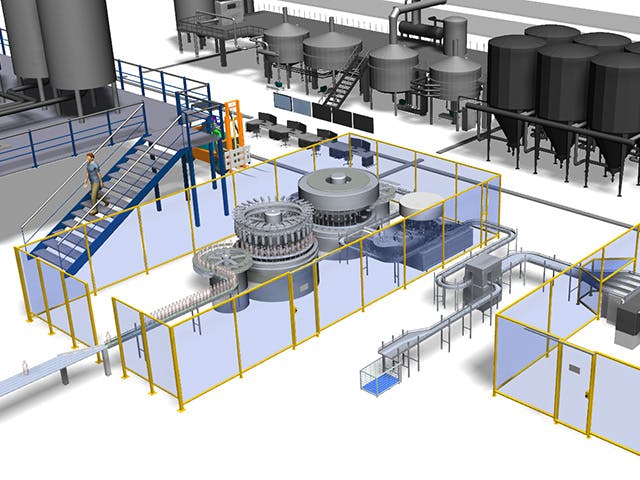
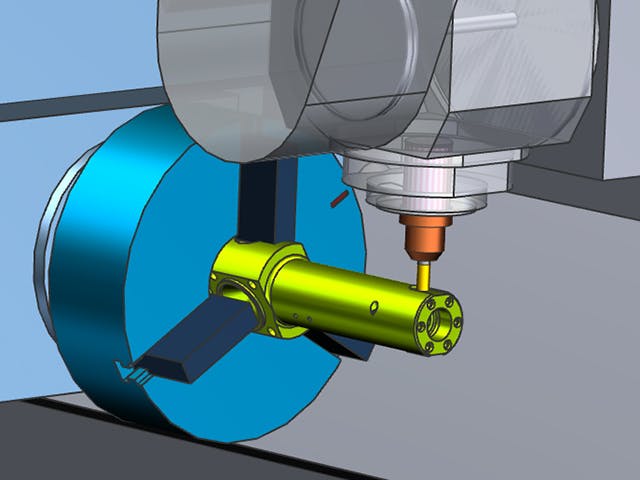
Digitale Fertigung ist die Verwendung eines integrierten, computerbasierten Systems, das aus Simulations-, 3D-Visualisierungs-, Analyse- und Kollaborationstools besteht, um gleichzeitig Produkt- und Fertigungsprozessdefinitionen zu erstellen. Die digitale Fertigung hat sich aus Fertigungsinitiativen wie Design for Manufacturability (DFM), computerintegrierter Fertigung (CIM), flexibler Fertigung und schlanker Fertigung entwickelt, die die Notwendigkeit eines kollaborativen Produkt- und Prozessdesigns hervorheben.
Viele der langfristigen Vorteile des Product Lifecycle Managements (PLM) können ohne eine umfassende digitale Fertigungsstrategie nicht erreicht werden. Die digitale Fertigung ist ein wichtiger Integrationspunkt zwischen PLM- und Fertigungsanwendungen und -anlagen und ermöglicht den Austausch produktbezogener Informationen zwischen Konstruktions- und Fertigungsgruppen. Diese Ausrichtung ermöglicht es Fertigungsunternehmen, Time-to-Market- und Volumenziele zu erreichen und Kosteneinsparungen zu erzielen, indem teure nachgelagerte Änderungen reduziert werden.
Verwandte Produkte: Tecnomatix | NX | Solid Edge