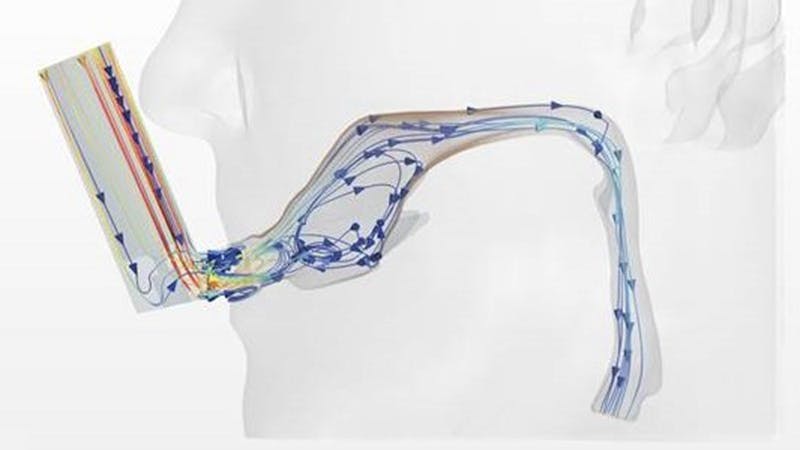
FDA uznává výhody, které nabízí modelování a simulace, a pravidelně výrobcům zdravotnických přístrojů doporučuje využívat simulační metodologie tak, aby bylo možné nové terapeutické prostředky vyvíjet bezpečným a efektivním způsobem od návrhu, přes předklinické studie a klinické testy až po uvedení na trh.
V tomto webináři Dr. Tina Morrisonová, zástupkyně ředitele divize aplikované mechaniky centra pro přístroje a radiologii Úřadu pro kontrolu potravin a léčiv, prezentuje přehled těchto metodologií a zdůrazňuje možnosti počítačových klinických testů, které lze použít k hodnocení zdravotnických výrobků.