Proměna každodenního života
Pojďme spolu transformovat dané odvětví a proměnit nápady na inovace.
Jak umělá inteligence mění údržbu továren
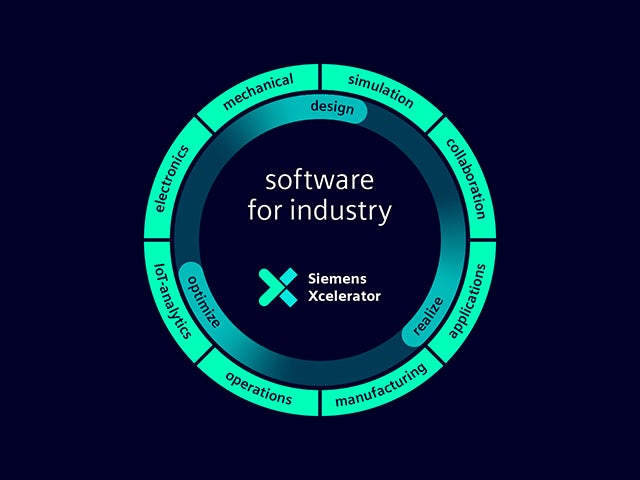
Naše digitální dvojče je jediným zdrojem důvěry, který umožňuje inteligentní rozhodování na základě dat. Pouze společnost Siemens spojuje reálný a digitální svět a vytváří tak skutečný digitální podnik.
Udržitelnost
Vytváříme udržitelné průmyslové inovace pro svět, ve kterém budeme chtít dnes i zítra žít, a pro průmysl, který si nepřeje naší planetě dále škodit.
Budoucnost mobility
Podporujeme budoucnost mobility ve vzduchu, na zemi i na moři a pomáháme vytvářet revoluci v dopravě, aby bylo cestování příjemnější a udržitelnější.
Aditivní výroba
Dodáváme pragmatickou aditivní výrobu pro společnosti všech velikostí. Podporujeme komplexní procesy aditivní výroby, aby byla snadná, nákladově efektivní a všudypřítomná.
Umělá inteligence
Naše inovace a řešení v oblasti umělé inteligence otevírají díky velkým objemům dat dveře novým obchodním příležitostem a podporují přijímání chytrých rozhodnutí, efektivnější práci, lepší kvalitu a rychlejší uvádění výrobků na trh.
Návrh systému založený na modelu
Naše metodika integrovaného návrhu systémů založeného na modelech (MBSE) sjednocuje všechny oblasti potřebné k vytvoření dnešních inteligentních systémů a všechny se zaměřují na urychlení inovace produktů nebo obchodního úspěchu.
Co je potřeba k proměně vaší společnosti na digitální podnik?
Společnost Surf Loch využívá dnešní digitální technologie, pomocí kterých způsobuje revoluci v oblasti surfingu. Malý tým specializovaných inženýrů této společnosti využívá software a služby portfolia Siemens Xcelerator a nabízí surfařům příležitost, jak sjíždět perfektní vlny, kdykoli budou chtít.
Přečtěte si další informace o inspirativní cestě této společnosti za digitální transformací.
Proměna každodenního života
Navštivte náš blog, kde najdete více informací o portfoliu Siemens Xcelerator a Siemens Xcelerator as a Service.